The 2nd GHWP (Guangzhou) Academy Training Notice
(3rd round notice)
I. Training Theme
“Innovative Medical Device Promotes Global Public Health”
Medical devices are a crucial foundation for healthcare and public health, directly affecting the safety and health of individuals. This training will focus on the innovation and development of high-end medical devices, including pre-market and post-market regulation of active medical devices. The training will focus on specific industry sectors, cutting-edge technologies, and regulatory practices. Frontline regulators and top experts will be invited to present.
II. Training Organization
Organizer: GHWP (Guangzhou) Academy
Co-organizer: Guangdong Association For Medical Devices Industry (GAMDI)
Supporting Organizers (Updating):
Guangdong Institute of Advanced Biomaterials and Medical Devices
China Association For Medical Devices Industry
Shenzhen Association for the Medical Devices
Acknowledgements: The Asia Pacific Medical Technology Association (APACMed)
Training Dates: November 23-27, 2024 (Registration on November 23, departure on November 27).
Training Place:Lecture Hall, 3rd Floor, Venture Capital Building, Longgang District, Shenzhen, Guangdong, China
「Venture Capital Building, 9 Tengfei Road, Longcheng Street, Shenzhen City, Guangdong Province」
IV. Training Methods and Content
Training Method: This program will combine lectures, penal discussions, Q&A sessions, case studies, hands-on training, and parallel exchanges. The morning session on November 24 will be available via live global streaming.
Training Content: Based on the needs collected by the GHWP (Guangzhou) Academy, five major training topics (Symposia) will be set, along with one hands-on training session, totaling 24 academic hours over three days. Each topic will include lectures and panel discussions.
- Training Topics
Based on the training needs collected by GHWP (Guangzhou) Academy, the Phase II training program includes three main topics and several parallel discussions.
- Topic 1: GHWP Promotion of Global Medical Device Regulatory Convergence, Coordination, and Trust (4 hours).
This topic will feature GHWP leadership introducing the progress of GHWP’s work in the fields of regulatory convergence, coordination, and trust in the global medical device sector.
- Topic 2: Regulation Requirements and Common Issues for Active Medical Devices in China (4 hours)
This session will invite authoritative experts from the National Medical Products Administration of China to explain up-to-date regulatory laws regarding active medical devices and relevant requirements for non-clinical and clinical data review.
- Topic 3: Innovation and Regulation in the Medical Robotics Industry (4 hours)
Focus: Surgical Robots and Rehabilitation Robots. This includes orthopedic, laparoscopic, and neurosurgical robots, exoskeleton robots for rehabilitation (upper and lower limbs), caregiving robots, and intelligent assistive mobility devices. Additionally, it covers therapeutic and rehabilitation equipment utilizing virtual reality, brain-computer interfaces, and other advanced technologies.
- Topic 4: Innovation and Regulation in the High-end Medical Imaging and Therapeutic Equipment Industry (4 hours)
Focus: Advanced Medical Imaging Diagnosis and Treatment Equipment and Technologies. This includes technologies for Computed Tomography (CT) devices (such as advanced X-ray tubes, flat-panel detectors, and other cutting-edge developments), Magnetic Resonance Imaging (MRI), tumor proton therapy systems, radiation therapy equipment, ultrasound devices (US), as well as auxiliary diagnostic technologies leveraging big data, artificial intelligence, and other innovative solutions.
- Topic 5: Innovation and Regulation in the Artificial Intelligence Medical Device Industry (4 hours)
Focus: Auxiliary diagnostic technologies such as big data and artificial intelligence, 5G-enabled medical devices, artificial intelligence-based medical devices, wearable medical devices, and digital therapeutic medical devices.
Topics 3, 4, and 5 will feature renowned international experts discussing current trends in technology innovation and industry development, with representatives from regulatory agencies of multiple countries and regions introducing pre-market and post-market regulatory requirements for high-end medical devices.
2. On-Site Training
Date: November 26, 2024, afternoon
Duration: Half a day
V. Target Audience
- Individuals engaged in the R&D of innovative medical devices, global expansion of the medical device industry, and international regulatory cooperation.
- Specifically invited representatives from medical device regulatory authorities and industry representatives of GHWP member countries/regions.
- Specifically invited members from governing bodies of the GHWP (Guangzhou) Academy.
- Specifically invited representatives from research institutions, clinical organizations, and associations.
The expected number of participants is approximately 300. The academy will create a valuable platform for communication between regulators from various countries and the industry.
After the training concludes, GHWP (Guangzhou) Academy will hold a graduation ceremony to award standardized completion certificates to the participants.
VI. Certificate of Completion
At the end of the training, the GHWP (Guangzhou) Academy will hold a ceremony to award participants with a unified completion certificate issued by the GHWP.
VII. Training Fees
Regulatory authorities of the GHWP members are entitled to 1-2 free training slots.
Members of the GHWP (Guangzhou) Academy Council will receive corresponding discounts according to the council's regulations.
Participants in the 1st training will enjoy a discounted fee.
Other participants will be charged RMB 9,800 per person, which covers tuition, materials, and meals (accommodation and transportation are self-arranged).

Payment Information:
Account Name: Guangdong Association For Medical Devices Industry
Account Number: 227807082179
Bank: Bank of Guangzhou, Yuexiu Branch
(Please indicate the participant's name + GHWP Phase II Training)
Training Meal Arrangements:
Dining location:HAIYUELIJING RESTAURANT
Hai Yue Li Jing Restaurant 「2nd Floor, Venture Capital Building 」
Dining arrangements: Lunch is available from 24 to 26 November
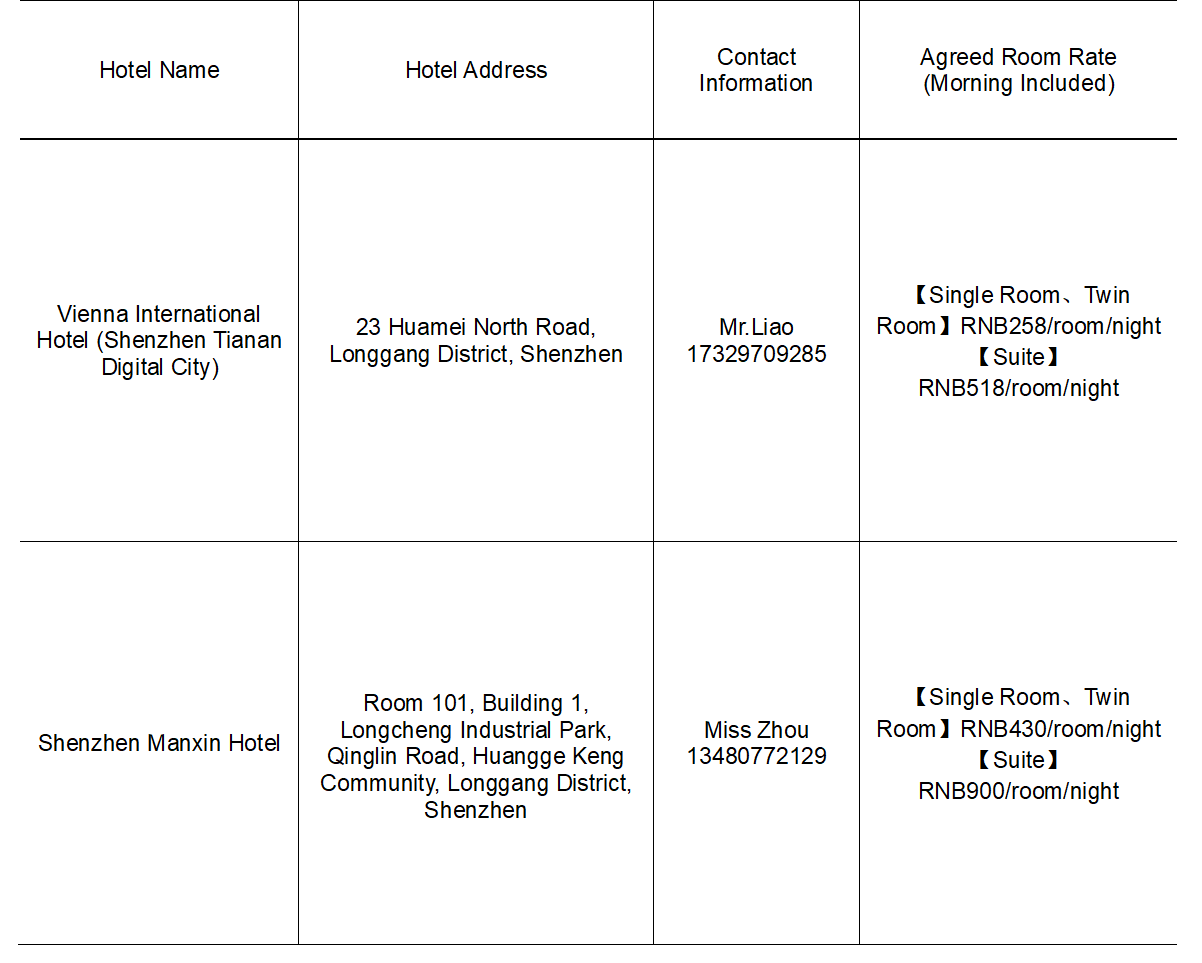
Protocol Hotel Information:
The training offers a hotel with a negotiated price. If necessary, you can contact the hotel for booking. This information is for reference only.
Ⅷ. Contacts
GHWP (Guangzhou) Academy: Ms. Sifan Wang, +86-13143128423, sylviawangsifan@ibmd.org.cn
Guangdong Association For Medical Devices Industry (GAMDI): Ms. Haiwei Ma +86-13632328457
Overseas participants, please fill out the attached registration receipt and return it to the conference email address. Others can scan the QR code to register online directly!

