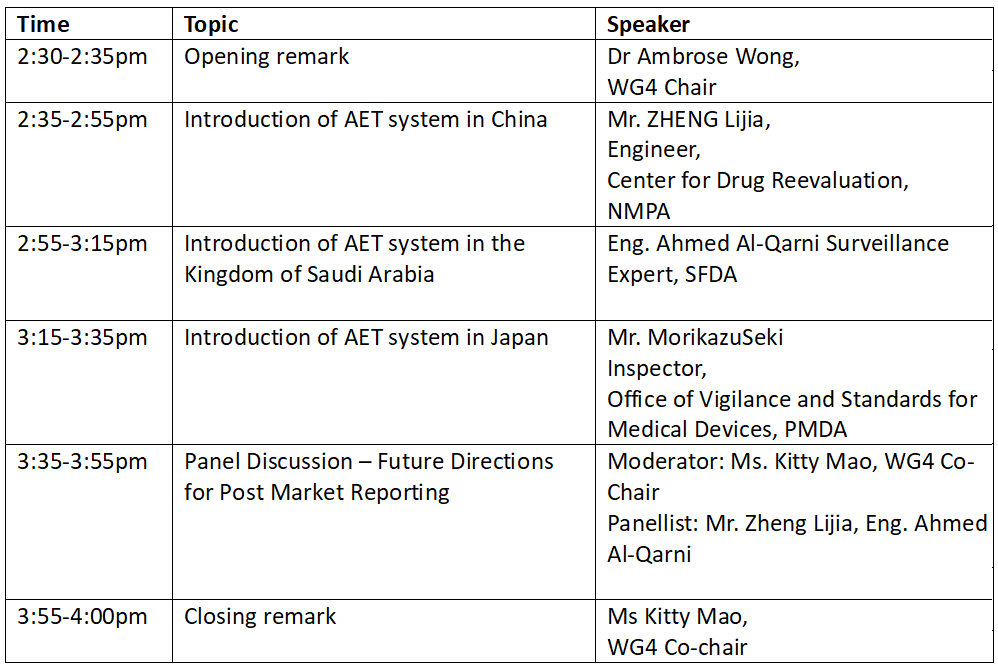
Capacity Building – Free online training on Medical Device Adverse Event Terminology and General Regulatory Requirements by NMPA, PMDA and SFDA Regulators on 20 November 2024
Time: 20 November 2024, 2:30-4:00 pm (GMT +8)
For All GHWP members
As part of the GHWP/GHWP Capacity Building program, we are pleased to announce an upcoming Free online training via Zoom on “Medical Device Adverse Event Terminology and General Regulatory Requirements”. This session will feature experts from NMPA, PMDA and SFDA Regulators.
In this training, we aim to provide an introduction of Medical Device Adverse Event Terminology systems in different jurisdictions and enhance participant’s understanding of general regulatory requirements. It will offer a platform for discussions on implementation and share implementation experiences, and encourage best practices.
No registration is required for this online training. Target audiences, regulators and industry who are interested, are welcome to join with the Zoom access as follows:
https://us06web.zoom.us/j/85905546420?pwd=QbQmLaZJarnd7GaJ9WVoo7BsDXEbV5.1
Meeting ID: 859 0554 6420
Passcode: 308674
Should you have any enquiry, please contact the GHWP Secretariat at secretariat@ghwp.info.